JIMCO’s environmentally friendly technology is know all over the world for purifying air and disinfect surfaces effectively with up 99,99%.
The process in JIMCO’s technology is called Photolytic oxidation, which is a combination of photolysis and ozonolysis. Both processes are needed to obtain the wanted effect
Photolysis is a photodecomposition process and is the effect of the UV-C radiation. Organic molecules (e.g., bacteria, germs, grease, fungal spores and virus) will be broken down by photons, when they are exposed to UV-C light. Ozonolysis is the oxidation process and is the effect of the natural ozone produced by the lamps.
The photo decomposed and broken organic molecules will be exposed to ozone and the ozone will make a cold incineration with the organic molecules and oxidize them. The photolytic oxidation process leaves no volatile compounds to the environment.
When cells are exposed to a UV rays in the range of 200–300 nm, it is absorbed into DNA, RNA, and protein. The absorption of UV destroys the cell wall and breaks down the DNA in the microorganism. When virus is exposed to UV-C the UV-C will destroy the outer protein coating which leads to the inactivation of the virus. When a virus is inactivated, it can no longer infect you.
Ozone is the world’s natural sterilizer. Ozone will effectively inactivate virus by destroying the envelope lipids, glycoproteins and capsid protein.
Different pathogens have different susceptibility and enveloped viruses tend to be far more susceptible compared to non-enveloped viruses (Naked viruses). Enveloped viruses have an envelope around the capsid and the envelope is normally easier to disrupt with heat, chemical disinfectant, UV and ozone.
Virus: A microorganism that is smaller than a bacterium that cannot grow or reproduce apart from in a living cell. A virus invades living cells and uses their chemical machinery to keep itself alive and to replicate itself. Viruses may contain either DNA or RNA as their genetic material

Most people know ultraviolet light from blue UV lamps or as radiation from the sun that you can protect yourself against with sunscreen lotion.
What many do not know is that there exist three categories of UV light:
UV-A, UV-B and UV-C.
The difference between the radiation types is decided by the wavelength measured in nanometers (nm).
UV-A is a part of the natural sunshine on the earth’s surface. The UV-A radiation causes different photochemical processes. It has a perceptible pigmenting – but in praxis no erythematic influence. Over a long time exposure, it can potentially cause physical damage, premature aging of the skin, indirect genetic damage and skin cancer.
UV-B radiation can be harmful to the skin and is the main cause of sunburn; excessive exposure can also cause cataracts, immune system suppression, and genetic damage, resulting in problems such as skin cancer- UV-B shows both pigmenting and erythematic influence. The ozone layer is very effective at screening out UV-B. Nevertheless, some UV-B, particularly at its longest wavelengths, reaches the surface, and is important for the skin’s production of vitamin D.
UV-C is naturally being produced by the sun and it is dangerous for humans if they are exposed to UV-C. UV-C is entirely screened out by the ozone layer and will never reach the Earth’s surface. UV-C has a strong germ fatal effect and can also cause erythema and conjunctivitis (see below).
UV ray comes from the sun. Most UV is absorbed into the ozone layer and the atmosphere, while 3%–4% arrive on the ground.
A total of 95% of the UV ray that arrives on earth is UV-A, and the remaining 5% is UV-B. UV-C, which has a powerful sterilizing effect on DNA,
is completely absorbed into the ozone layer and the atmosphere.
For a long time, we have been aware that the sun may prevent the possibility for bacteria to spread.
In 1877, the two English researchers Downes and Blount (Downes, A., Blount T.P.: Researchers on the effect of sunlight upon bacteria and other organisms, London: Proc. Royal Society, 1877) found out that the breeding of micro-organisms stopped when these were exposed to the sunlight.
At the time, it was not yet possible to explain the process involved. Later research has shown that the effect mentioned comes from the invisible part of the radiation of the sun that is below 320 nm.
When this knowledge became evident, it was possible to develop an artificial source of radiation for destroying bacteria.
This led to new possibilities in the area of disinfection of air as well as surface disinfection of solid material.
Today, disinfection with UV-C light is not only very valuable but also a necessity as a supplement to other disinfection methods.
JIMCO like to make a smart air purification solution for everyone, and by the result from the UV-C effect we continue innovating, so you can have the best UV-C & Ozone air purification treatment as possible.
As confirmed by numerous researches and reports, when a cell is exposed to a UV ray in the range of 200–300 nm, it is absorbed into DNA, RNA, and protein.
Such protein absorption destroys the cell wall and eliminates the DNA function, resulting in sterilization of the organism.
The UV ray is absorbed into thymine, one of the four bases (adenine, guanine, cytosine, thymine) that connect the double helix structure of the DNA,
to deactivate the double helix structure. When this process occurs in the DNA, it hinders the formation of protein to stop the DNA cloning process, which in turn,
leads to no cell being replicated. This reaction is a light reaction that occurs in an instant, faster than other sterilizing methods (chemicals or high heat) and leaving no residue.
Most people know ozone from the ozone layer in the Earth’s stratosphere, but ozone also exists in the Earth’s atmosphere.
Ozone is a vital part for the existence of human life on Earth because ozone in the ozone layer is screening out dangerous radiation from the sun and because ozone in the troposphere is oxidizing bacteria, viruses, algae and fungi. Natural ozone near the Earth’s surface plays an important beneficial role in chemically removing pollutants from the atmosphere.
Ozone is a colorless unstable gas with a pungent odor. The best way to describe the smell is the smell in the air after rain and lightning. Some will describe the smell as fresh, and others cannot stand it.
High concentration of ozone is hazardous to humans and hence as all hazardous gases subdued to a threshold value of 0.1 ppm – corresponding to 0,22 mg O3/m3 air.
The Danish Work Authorities recommend that the working environment does not exceed 0.1 ppm. By that recommendation it is possible for JIMCO to give you an UV-C & Ozone air purification treatment that follows these recommendations.
An ozone molecule (O3) consists of three oxygen atoms and can be made when ordinary oxygen molecules (O2) are exposed to energy. Energy will split the ordinary oxygen molecules (O2) leaving two oxygen atoms (O). The atomic oxygen then combines with unbroken ordinary oxygen molecules (O2) to create ozone (O3).Chemically, this can be described as:
Ozone reverts back to oxygen after oxidizing with organic matter. If the room is sterilized and there are no particles to react with ozone will revert back to oxygen due to heat. At room temperature ozone’s half-life is 3 days and at 120°C it is 28 minutes.
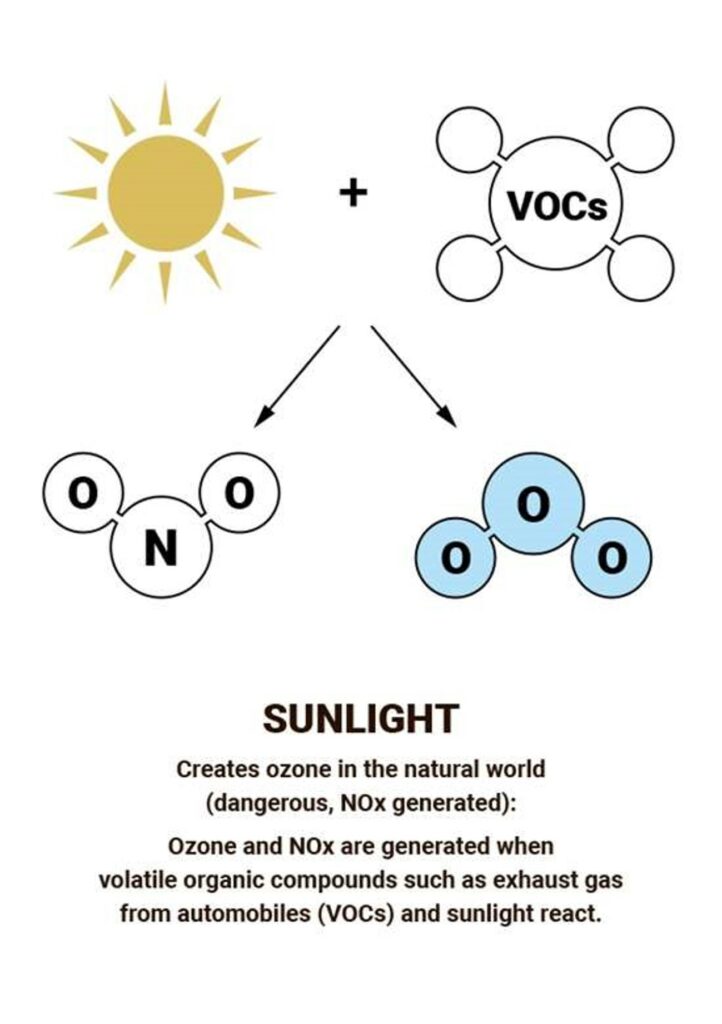
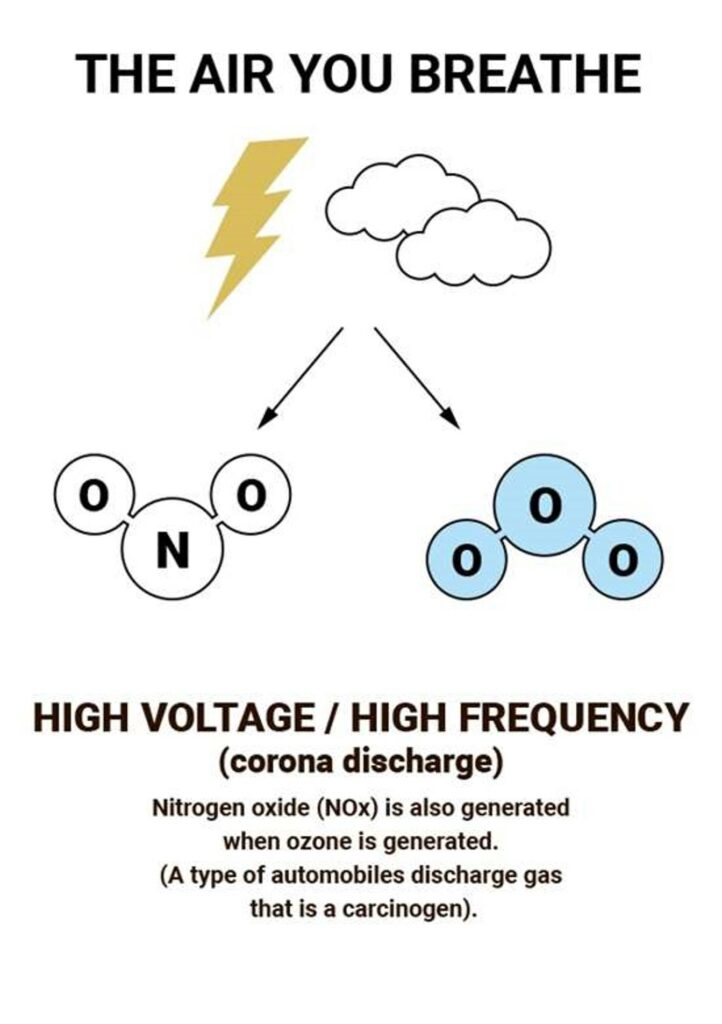
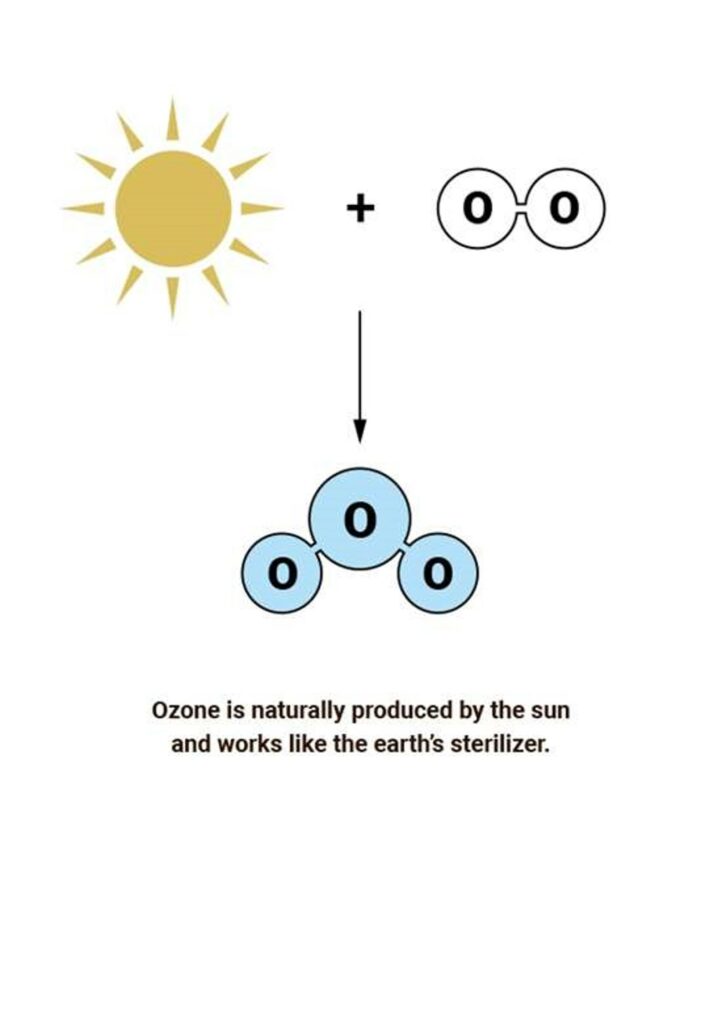
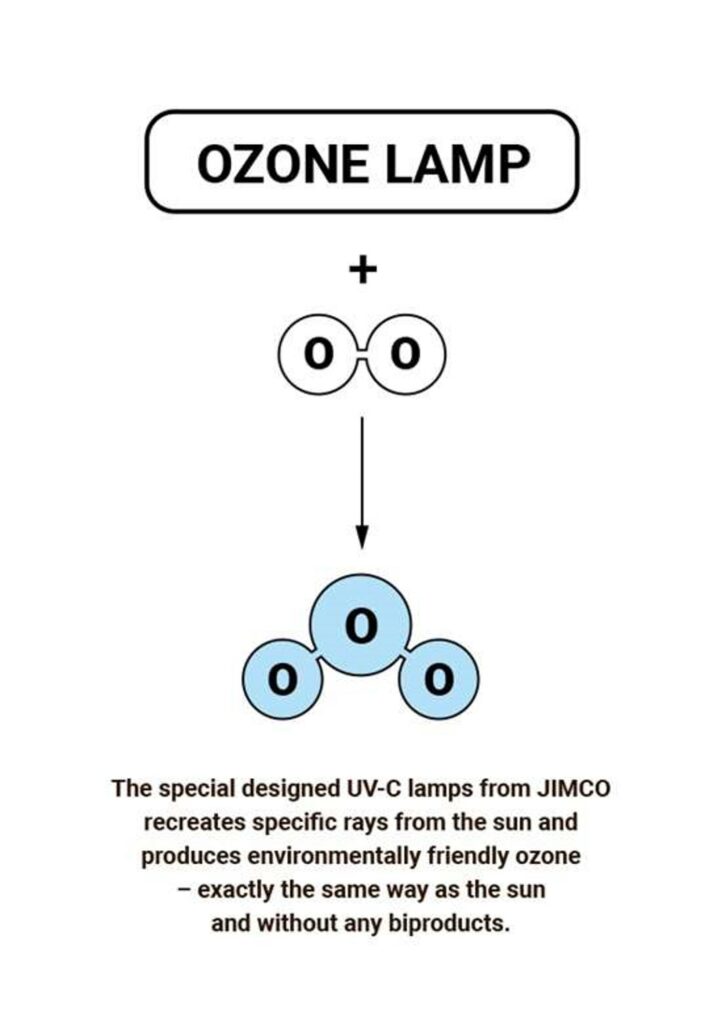
Most ozone (about 90%) is found in the stratosphere, which begins about 10 kilometers above Earth’s surface and extends up to about 50 kilometers altitude. The stratospheric region with the highest ozone concentration is commonly known as the ozone layer.
The ozone layer extends over the entire globe with some variation in altitude and thickness. The remaining ozone, about 10%, is found in the troposphere, which is the lowest region of the atmosphere, between Earth’s surface and the stratosphere.
The ozone layer contains less than 10 parts per million (ppm) of ozone, while the average ozone concentration in Earth’s atmosphere as a whole is about 0.3 parts per million (ppm).
Ozone in the Earth’s stratosphere is created by ultraviolet light striking ordinary ozygenmolecules containing two oxygen atoms (O2), splitting them into individual oxygen atoms (O). The atomic oxygen then combines with unbroken ordinary oxygen molecules (O2) to create ozone (O3). The UV light from the sun act as the energy source in this process.
Ultraviolet radiation with wavelengths 100-200 nm forms ozone (O3) from oxygen molecules (O2). That is how the JIMCO UV-C & Ozone air purification treatment is formed.
Although the concentration of the ozone in the ozone layer is very small, it is vitally important to life because it absorbs harmful ultraviolet (UV) radiation coming from the sun.
The ozone in the ozone layer is screening out all UV-C radiation coming from the sun, preventing it from reaching the Earth’s surface. Once UV-C strikes an ozone molecule, it will decompose the ozone molecule. In that process the ozone molecule will revert back to an oxygen molecule (O2).
In an unpolluted atmosphere there is a balance between the amount of ozone being produced and destroyed and so the total concentration remains relatively constant. This continuing process is referred to the ozone-oxygen cycle.
At different temperatures and pressures (i.e. varying altitudes), there are different production and destruction reaction rates leading to a variation in concentration. The highest ozone concentrations are in the lower stratosphere, between about 18 and 26 km.
In our opinion and experience, UV-C and Ozone offer many safer alternatives to sanitation, disinfection, grease, fat, and odor removal as well as eliminate and reduce mold, bacteria, and viruses if used correctly.
The links provided below are intended solely to enhance the knowledge of the usage as well as the benefits and disadvantages of using UV-C and Ozone as a safer and more environmentally friendly alternative to chemicals such as Chlorine, hydrogen peroxide Alcohol, Formaldehyde, Glutaraldehyde, Iodophors, Ortho-phthalaldehyde (OPA), Peracetic acid, Peracetic acid, hydrogen peroxide, Phenolics, and Quaternary ammonium compounds.
Please consult an expert before installing, using, or testing any UV-C and Ozone generating devices to ensure the necessary health and safety precautions are taken.
Cleaning the air with ultraviolet germicidal irradiation lessened contact infections in a long-term acute care hospital
By: Tina Ethington MSN, RN, CEN, NE-Bca, Sherry Newsome BSN, RN, MBA/MNAa, Jerri WaughBSN, RN, MBA/MHAa, Linda D. Lee DrPH, MBAb
Fast inactivation of SARS-CoV-2 by UV-C and ozone exposure on different materials
By: Elena Criscuolo, Roberta A. Diotti, Roberto Ferrarese,Cesare Alippi,Gabriele Viscardib , Carlo Signorelli, Nicasio Mancini, Massimo Clementi, and Nicola Clementi
Airborne Microorganism Inactivation by a UV-C LED and Ionizer-Based Continuous Sanitation Air (CSA) System in Train Environments
By: Giulia Baldelli, Mattia Paolo Aliano, Giulia Amagliani, Mauro Magnani, Giorgio Brandi, Carmelo Pennino, Giuditta Fiorella Schiavano
Effect of low-dose gaseous ozone on pathogenic Bacteria
By: Belchor Fontes, Ana Maria Cattani Heimbecker, Glacus de Souza Brito, Silvia F Costa,Inneke M van der Heijden, Anna S Levin and Samir Rasslan
Application of Ozone in Food Industries
By: L. VIKAS AND S.V. LAKSHMINARAYANA
Inactivation of Coronaviruses in food industry: The use of inorganic and organic disinfectants, ozone, and UV radiation
By: Roberto Quevedo-León, José Bastías-Montes, Teófilo Espinoza-Tellez, Betty Ronceros, Iván Balic, Ociel Muñoz
Decontamination of chilli flakes in a fluidized bed using combined technologies: Infrared, UV and ozone
By: Ian Watson, Prashant Kamble, Callum Shanks, Zakir Khana, Nada El Darra
Development of Ozone Technology Rice Storage Systems (OTRISS) for Quality Improvement of Rice Production
By: M Nur, E Kusdiyantini, W Wuryanti, T A Winarni, S A Widyanto and H Muharam
Different Uses of Ozone: Environmental and Corporate Sustainability. Literature Review and Case Study
By : Marco Remondino and Luigi Valdenassi
Extension of Fish Shelf Life by Ozone Treatment
By: Behrouz Mosayebi Dehkordi, and Neda Zokaie
Inactivation of microbes by ozone in the food industry
By: Mohamed Ziyaina and Barbara Rasco
Use of ozone in the dairy industry
By: László Varga,Jenő Szigeti
Ozone – an Emerging Technology for the Seafood Industry
By : Alex Augusto Gonçalves
Ozone Contribution in Food Industry in Japan
By: Shigezo Naito1and Hirofumi Takahara
The Application of Ozone Technology for Public Health and Industry
By: Laurence Franken, M.S.
Air disinfection and food preservation by ozone gas
By: Nguyen Hoang Nghi, Le Cao Cuong, Tran Vinh Dieu, Doan Thi Yen Oanh
Study of ozone disinfection in the hospital environment
By : Le Hoang Tu, Le Hoang Oanh, Nguyen Vu Trung, Le Cao Cuong, Doan Thi Yen Oanh, Tran Vinh Dieu, Nguyen Hoang Nghi
The Effect of Ozone on Common Environmental Fungi
By: William Korzun, Jeffery Hall and Ronald Sauer
Improving Indoor Air Quality Through The Use of Ultraviolet Technology In Commercial Buildings
By: Daniel de Robles, P.E., Scott W. Kramer, Ph.D
Recent case studies on the use of ozone to combat coronavirus
By: Soumya Nagashri Manjunath, M. Sakar, Manmohan Katapadi and R. Geetha Balakrishna
You can also learn more about UV-C & Ozone here.

